-
article · 2026Year54Moon31Day
Flex蛋白质组学工作站的热振荡模块如何加速蛋白质检测
Read More -
article · 2026Year48Moon30Day
Flex蛋白质组学工作站如何帮助加速蛋白质样品分析?
Read More -
article · 2026Year9Moon29Day
Flex蛋白质组学工作站的功能与优势
Read More


Modern data analysis methods, such as optimization algorithms or deep learning, have been successfully applied to many biotechnological and medical problems. For these methods to be effective, a large number of high-quality and reproducible experiments are required, which require a high degree of automation. Here, we introduce an open source hardware and low-cost framework for automated high-throughput generation of large amounts of cell biology data. Our design includes an epifluorescence microscope with an automated XY stage for moving multiwell plates containing cells, and a perfusion manifold that allows for the programmable application of up to eight different solutions. Our system is extremely flexible and can be easily adapted to individual experimental needs. To demonstrate the utility of this system, we have used it to perform high-throughput Ca 2+ imaging and large-scale fluorescent labeling experiments.
Introduction Deep learning and artificial neural networks (ANN) developed over the past decade have proven useful in image analysis, optimization tasks, and robotics (LeCun et al., 2015; Hinton, 2018; Hinton et al., 2019). They are also increasingly popular for solving biological problems. For example, ANN-based cell segmentation algorithms are more accurate and faster than traditional methods ( Hilsenbeck et al., 2017 ). Deep learning also helps detect transformed cells in human tissues (Van Valen et al., 2016; Coudray et al., 2018), optimize treatment conditions (Kusumoto and Yuasa, 2019), and interpret animal behavior (Heras et al., 2019). Recently, an online platform has been developed that allows researchers without any deep learning knowledge to use it in their own applications (von Chamier et al., 2020), further increasing the usefulness of deep learning as an analytical tool.
An important consideration when applying deep learning and other machine learning methods is the size of the training data set. Typically, deep learning requires thousands to tens of thousands of data points (O'Mahony et al., 2019). This is usually not feasible in biological experiments because they often take a long time to complete. Therefore, there is a need for automated systems that can perform hundreds or thousands of experiments with minimal human supervision. Such an automated system should allow (a) single cell microscopy (brightfield and/or fluorescence) in multiple wells (i.e. with an XY stage); (b) automated application of multiple different solutions; (c) ) Automated online analysis (e.g., cell segmentation and calculation of average brightness).
Commercially available fluorescence microscopes (such as the Olympus BX61, Nikon Ti Widefield or Nikon A1 confocal systems, and live cell imaging systems such as the Echo Revolve or Sartorius Incucyte) are often equipped with XY stages for multi-well fluorescence imaging. However, these systems are expensive (£15,000-150,000) and offer only limited capabilities for the application of automated solutions. Many commercially available systems (e.g., Hamilton or Andrew) allow this, but due to their size and cost, these systems are difficult to integrate with live imaging.
The development of 3D printing and cheap electronics like Arduino and Raspberry Pie has sparked a revolution in customizing affordable scientific equipment that was previously only available in large labs or university facilities. Not only are these devices affordable, they can be customized to suit individual laboratory needs. An example of such technology is labware.net developed by Baden et al. (2015) and Maia Chagas et al. (2017), which allows 3D printing of extremely cheap laboratory equipment, ranging from standard available micropipettes and Operators to fluorescence microscopy and optogenetic solutions.
Several open source high-quality microscopes have recently been developed. For example, Diederich et al. (2020) developed a customizable 3D-printed open source framework that can be used to build a variety of microscopes: from simple brightfield microscopes with autofocus to more complex systems with optical sectioning of samples. However, these resources lack open source systems for scanning large numbers of samples. Sharkey et al. (2016) addressed this issue for small movements, and Merces et al. (2021) addressed this issue for robust imaging of multi-well plates. However, these solutions do not provide any cellular manipulation, although open source liquid handling solutions have recently been developed (e.g., Wijnen et al., 2014; Almada et al., 2019; Amarante et al., 2019; Booeshaghi et al., 2019 year; Samokhin, 2020; Baas and Saggiomo, 2021).
Here, we introduce an open source system that combines high-throughput microscopy in multi-well plates, automated solution application, simultaneous fluorescence imaging, and image analysis. It is low cost (£400-600 without fluorescence microscope and £2,500 with fluorescence microscope), fully customizable, and can run up to 96 or 384 experiments consecutively if the experiment does not require high sampling rates (e.g. 1 frame per minute or higher), you can do both at the same time. The platform is equipped with a single-channel epifluorescence microscope lens that can be used to image dynamic fluorescent reporters (e.g., GCaMP and synthetic calcium dyes; Razlivanov et al., 2018 ) and/or samples labeled with fluorescent antibodies or dyes. We demonstrate how our system can rapidly generate cellular biological data at scale and under tightly controlled and reproducible experimental conditions.
Results Typical cell biology experimental paradigms often involve treating cells with bioactive compounds (e.g., growth factors, calcium mobilization agonists, and cytotoxic agents) and monitoring cell behavior using fluorescent reporters or fixing cells for subsequent immunofluorescence labeling or genetic testing. Expression analysis. To automate such experiments, we developed an experimental platform that allows automated imaging of 96-well plates and application of eight solutions using a syringe pump. We first introduce the platform and demonstrate its applicability, then describe the hardware, software, and system performance in more detail.
The fully assembled system of the automation platform and its suitability is shown in Figure 1A. The hardware consists of several main modules: an XY stage that moves the multi-well plate horizontally; a small fluorescence microscope with an autofocus system (Supplementary Video 1); and a perfusion manifold that applies eight solutions to individual wells (Supplementary Video 2) . Build instructions and all 3D printing files are available at https://github.com/frescolabs/FrescoM (see also supplementary text). The hardware can be operated using a user interface written in Python (Figure 1B, see "Software" section for details), which controls the platform, objectives, and manifolds as well as the overall management of autofocus, exposure, illumination, and experimental protocols.
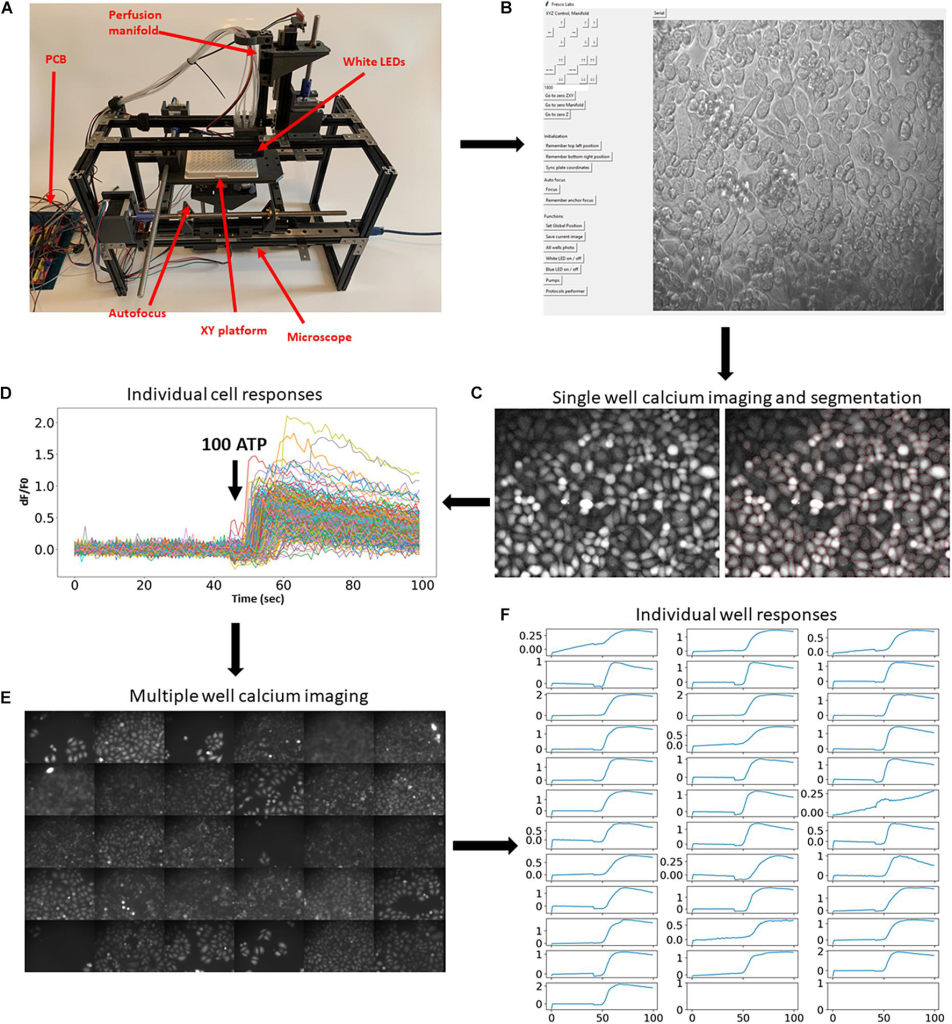
To demonstrate the platform, we first demonstrated how it can be used in large-scale fluorescence imaging experiments (Nasu et al., 2021). Typical experimental protocols involving fluorescent reporters require imaging of cells for a period of time before and after the application of agonists, growth factors, or other active compounds. To demonstrate the usability of the developed experimental platform for this experimental paradigm, we performed calcium imaging using a synthesized fluorescent indicator of calcium concentration. Cells were automatically labeled with the fluorescent calcium dye Fluo4-AM (Fig. 1C ) and subjected to calcium imaging in response to 100 μ m ATP. Cells were then automatically segmented using the Cellpose algorithm (Stringer et al., 2021) (Figure 1C, right), and the fluorescence dynamics of individual cells were extracted (Figure 1D). The same experimental procedure and analysis were then automatically repeated in the other 30 wells (Figure 1E,F and Supplementary Video 3). These experiments demonstrate that the developed platform enables robust and automated high-throughput imaging of fluorescent reporters.
Another important advantage of the developed system is that it allows large-scale generation of images in a large number of wells. To demonstrate this usability, we generated a Python protocol class (Supplementary Protocol 1, see Software section for details) that moves the platform over all 96 wells, focuses on each cell, and captures bright field or fluorescence image. Examples of such experiments are shown in Figures 2A,B. Importantly, this system allows scanning a single well and generating multiple images of the same well (Figure 2C), which is useful for finding rare cells (e.g., cells undergoing mitosis/apoptosis or positive cells with inefficient transfection). it works.

To demonstrate the usability of the developed experimental platform in labeling experiments, we used fluorescent wheat germ agglutinin (WGA), which highlights cell membranes. Five rows of wells (48 wells in total) were automatically washed with PBS and then a solution containing 5 mg/ml fluorescent WGA was added. After standing at room temperature for 10 minutes, the WGA was washed with PBS and then placed under a fluorescence microscope for observation. The resulting fluorescence image is shown in Figure 2D. Automated labeling produced clear images of cells with well-defined plasma membranes, demonstrating that routine labeling procedures can be automated using the developed platform.
Finally, we also tested whether the developed platform could be used for immunofluorescence staining with anti-HER2 antibody (Herceptin) as primary antibody and Alexa 488-conjugated secondary antibody. Wells containing SKBR3 cells (high expression of HER2) or HeLa cells (low expression of HER2) were automatically perfused with PBS and Herceptin. After incubation for 40 min at room temperature, cells were perfused with PBS followed by secondary antibodies. After an additional 30 min of incubation at room temperature, the wells were perfused with imaging solution and fluorescence imaging was performed (Figure 2E and Supplementary Figure 1). The resulting images show clear labeling of the cell membranes of SKBR3 cells with high HER2 levels but unclear labeling of HeLa cells with low receptor levels, demonstrating the robustness of the automated labeling procedure.
These examples demonstrate the broad usability of the developed experimental platform in automating different types of cell biology experiments. Below, we describe the platform in detail and demonstrate its performance in a series of tests.
Hardware Design The overall structure is constructed from MakerbeamXL 15 mm × 15 mm extrusions, connected via L- and T-shaped aluminum brackets or 3D printed parts. We found that using an aluminum extrusion frame instead of a fully 3D printed part (both models available at https://github.com/frescolabs/FrescoM) made the entire system more stable and reduced vibration (data not shown). The frame consists of eight side extrusions (four vertical 300 mm and three horizontal 200 mm, two at the top and one at the bottom, Figure 3A). The sides are attached to two 400mm extrusions that hold the MGH12 rails that drive the x-axis. The y-axis (Figure 3B) rests on a square frame constructed of four 200 mm extrusions and is connected to the x-axis via two 3D printed brackets. Two MGH12 rails are located on the frame to accommodate a Nema 17 motor and a 96-well plate holder (Figure 3B, y_plate_holder.stl file).
The experienced service team and strong production support team provide customers with worry-free order services.

简体中文

繁體中文

English

日本語

한국인