-
article · 2026Year53Moon5Day
Flex蛋白质组学工作站的磁力模块:提升蛋白质纯化效率
Read More -
article · 2026Year22Moon4Day
利用Flex蛋白质组学工作站实现高效的蛋白质纯化与酶解消化
Read More -
article · 2026Year54Moon31Day
Flex蛋白质组学工作站的热振荡模块如何加速蛋白质检测
Read More


1 Introduction The life sciences are in the midst of a “reproducibility crisis” due to concerns about data reliability and the inability to reproduce published research results. Most researchers report being unable to reproduce experiments (including their own), so more steps need to be taken to improve the reliability and credibility of published research results. But what factors contribute to this scientific “crisis” and how can simple pipetting solutions be implemented to combat it? This white paper outlines the extent of the reproducibility crisis, explores the five main factors that contribute to unreproducible experiments, and discusses how good pipette selection and technique can avoid poor reproducibility caused by inaccurate liquid handling. question.
2 Data Reproducibility Crisis Reproducibility is not just an artifact of a specific time, place, or person. It is the cornerstone of the scientific method and a necessary condition for confirming whether the observed results are consistent with the hypothesis. The scientific community relies on a peer-reviewed publication process to ensure that scientific research is accurate, which means that on the face of it, most research results are rarely questioned once they are published. As a result, these studies are rarely replicated, published findings are accepted as fact, and a field of research may be based on an irreproducible finding. Our current focus on reducing the use of animals in scientific research (such as the “3 R’s” framework for more humane animal research through animal replacement, animal reduction, and research improvement) further exacerbates this phenomenon. This framework recommends prioritizing research that “really enters the knowledge base,” but does nothing to promote the reproduction of existing knowledge. However, if a specific research result cannot be reproduced by different people in different circumstances, we cannot regard the result as a true scientific phenomenon, but only as a product of the specific experimental conditions.
The reproducibility crisis in (bio)medicine became the subject of a controversial 2005 journal article by John Ioannidis titled “Why Most Published Research Results Are Wrong” (1) focus of attention. Simulation and modeling results indicate that for most study designs, published research results are likely to be erroneous and are simply accurate measurements under current prevailing biases.
The problem of data reproducibility was raised nearly two decades ago and remains prevalent today. A recent survey by Nature showed that more than 70% of scientific researchers reported being unable to reproduce published experimental results (2). Worryingly, more than 50% of researchers reported being unable to reproduce their experiments. It is now agreed that this problem is so serious that it has led to a reproducibility crisis in the fields of biology and medicine. Most researchers said that although they agreed with this view, they believed the published literature.
The reproducibility crisis not only disrupts the knowledge base of basic science but also severely affects development plans for industrial applications. Clearly, we need to leverage solutions to improve data reliability and address the reproducibility crisis in science, but what factors contribute to a lack of reproducibility?
3 Factors affecting data reproducibility To deal with the reproducibility crisis, we must first admit that there are data reproducibility problems in the scientific community, and then consider and understand the main factors that affect data reliability. planning to experimental procedures and final analysis) take steps to avoid these factors.
3.1 Culture Arguably, the main reason for not perceiving the irreproducibility of research is that the current scientific culture discourages the publication of negative research results. Researchers who are unable to reproduce an experiment often do so because they did not perform the experiment correctly, lack the necessary skills, or because the available protocols do not have enough detail to support reproducing the experiment. However, journals may be reluctant to publish negative findings even after numerous lab members deem the experiment irreproducible after repeated attempts. Even if published, negative research results are often viewed as controversial and refuted. As noted in Nature's findings, "Several respondents who had publicly published results that failed to replicate experiments reported that editors and reviewers requested comparisons between these studies and the original study." But established journals recognize the problem. , making it easier than ever to publish negative research results, including a collection of articles in the special issue of the journal PLOS One titled "The Missing Pieces: Negative, inconclusive and inconclusive findings" (3).
3.2 Emphasis on P Values The high rate of non-reproducibility in studies is most likely due to researchers claiming conclusive results based on statistical significance observed in a single study (usually P values less than 0.05). The gold standard of significance P < 0.05 may also lead researchers to incorrectly interpret wrong results. This standard does not necessarily confirm that the research results are practical and important, nor does it indicate whether the data will be consistent when the same experiment is performed elsewhere. Still significant. Relying on statistical significance to determine whether a study is worthy of publication has led researchers to engage in “p-value manipulation” (4). Such malicious behavior may include designing and conducting experiments to ensure significant results or leveraging data analytics through data dredging, data fishing, data mining, or data capture.
3.3 Lack of transparency and raw data Only in recent years have there been incentives to provide researchers with full access to the raw data behind their studies. However, in the biomedical field, where research results can generate enormous wealth through commercialization, there is still a lack of transparency into exactly how data are collected and analyzed. To be honest, journal articles have word limits, making it difficult in many cases to include all the methodological details needed to successfully reproduce complex experiments. In other areas where commercial or intellectual property considerations are a concern, proprietary information may be removed or obfuscated in the manuscript. If the raw data themselves provide a valuable product (such as a genomic database influencing drug development), these data are often kept confidential for commercial reasons. Therefore, outside researchers cannot independently verify published study results and thus determine whether the results are reproducible and conclusive.
3.4 Poor laboratory practices and record keeping At a more basic level, non-reproducible experiments may be due to poor laboratory skills on the part of the original experimenter and the repeater experimenter, who should apply the technology in a standard way whenever possible. So that other researchers can use these technologies. If a new technology is developed for a specific scenario (which may not be understandable through a text scenario), a video tutorial can be included. Small steps may be overlooked and not included in the published protocol, so reproducibility may also be affected if not documented in detail.
3.5 Non-standard equipment varies in quality The variety of laboratory equipment and instruments available can also affect protocol standardization. The overall functionality of instruments from different brands may be similar, but their accuracy may vary. Differences in sensitivity, specificity, detection level, etc. between different brands may have a significant impact on experimental results. Therefore, a detailed list of materials and equipment and a published plan are required, clearly stating the brand, model, batch number and other information of the items used. Quality equipment and materials should be used wherever possible to reduce differences that may be associated with cheaper materials. These problems can be further exacerbated by uncalibrated equipment and changes in experimental parameters without the knowledge of the researcher.
4 Improve reproducibility with easy-to-implement pipetting solutions The reproducibility crisis is a systemic problem that requires consideration and solutions at many stages of the scientific method, but improving data can start with improving simple processes. reliability. Improving processes involving multiple scientific workflow stages can lead to high returns by increasing data reliability.
Our mission is to create reliable and easily verifiable high-precision instruments to help researchers achieve reproducible science. Manual pipetting is an easily overlooked life science tool. This tool requires accuracy down to microliters. It can be said that it is used frequently in all processes, so it is a key process to improve reproducibility. Fortunately, pipetting solutions are simple and affordable, making a positive impact throughout your benchtop workflow.
4.1 Choose a high-quality pipette To achieve reproducible pipetting, start by choosing a high-quality pipette that is accurate throughout the entire pipetting cycle (aspiration and dispensing). The true value (also called accuracy) of a pipette is the ability to aspirate and dispense solution volumes close to the true or nominal value indicated by the volume setting. Pipette precision refers to the ability to dispense the same volume of solution over multiple repeated operations. Therefore, the overall pipetting performance of a pipette can be achieved by balancing high true values and/or high precision to achieve low systematic errors and/or low random errors, respectively.
After choosing a quality pipette, there are still several factors that can negatively impact accuracy:
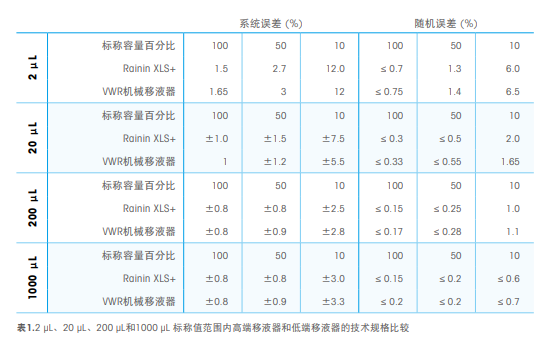
The selected pipette should use advanced manufacturing processes to achieve tolerances that maintain long-term accuracy (true value and precision). When purchasing a new pipette, the stated systematic error specifications and random error specifications should be compared between vendors. To illustrate the differences between brands, Table 1 compares technical specifications between high-end and low-end pipettes in the 2 µL, 20 µL, 200 µL, and 1000 µL nominal ranges. Overall, high-end instruments have better random error performance. In addition, special attention should be paid to low-volume pipettes—that is, those with a dispensing capacity of less than 10 µL—as pipetting technique and sample viscosity have a greater negative impact on low-volume dispensing, resulting in reduced accuracy and expected volume reduction. Small. Therefore, users should choose pipettes with smaller instrument errors when shipped from the factory.
For molecular workflows that require high precision and repeatability (such as reverse transcription-polymerase chain reaction, RT-PCR), the relatively low precision of inferior instruments can have a significant impact on the reproducibility of experiments.
4.2 Ensure routine maintenance, calibration and performance verification are performed correctly Pipettes are widely used equipment in laboratories and are subject to constant wear and tear. Pipettes are made up of multiple mechanical parts that move around each other and are often exposed to corrosive or harmful chemicals. Like all other mechanical products, pipette parts deteriorate over time, seriously affecting pipette performance. High-end pipettes and instruments are guaranteed to outlast lower-priced alternatives, but they still require regular maintenance, validation, and calibration to ensure consistent performance and reproducibility across experiments.
Pipette Maintenance Pipette maintenance includes routine care and preventive maintenance. Proper care of your pipettes will maximize performance between repairs and prevent pipette damage that renders it unusable. Simple daily activities can be performed to ensure that the pipette is working properly.
Check your pipette regularly for any visible signs of damage, such as scratches or breaks in the pipette housing. This activity can indicate if the pipette has been dropped, which could cause damage and require repair or calibration.
Clean pipettes regularly to prevent dust or dirt from getting into the workpiece and causing it to rub or jam. Clean the pipette using a non-abrasive cleaning solution recommended by the manufacturer, and then wipe the pipette with a lint-free cloth.
Disinfect pipettes properly (limit exposure to UV light to 10-15 minutes) so that the integrity of the pipette housing is not compromised. Continuous autoclaving results in pipettes being frequently exposed to high temperatures, pressures, and humidity, which may also affect their performance, so performance verification should be performed after each autoclaving.
Store pipettes securely in a suitable box or platform to prevent them from falling and damage.
Never invert the pipette (during liquid handling) to avoid liquid entering the instrument. Filter tips help prevent liquids and contaminants from entering the pipette.
Routine pipette care can help maintain pipette performance between service intervals, but preventive maintenance is still critical to prevent sudden pipette failure. Rainin service data shows that if no preventive maintenance is performed or regular repair procedures are performed, pipette failure rates can reach 20% or more, even with annual inspections. About 95% of pipette failures are due to sealing system defects, which can be predicted and prevented. Preventive maintenance is designed to replace worn components (such as O-rings, seals, and plungers) before they fail and further damage surrounding components.
Based on the manufacturer's recommendations and our extensive experience, under normal use conditions, seals should be replaced at least once a year, while tip holders and pistons should be replaced every three to five years. Preventive maintenance helps ensure that pipette performance is maintained, prevents pipette failure, and keeps experiments compliant and reproducible, thus avoiding workflow disruptions.
Calibration recommends preventive maintenance at least once a year to ensure proper pipette functionality, but the pipette needs to be calibrated more frequently to ensure its accuracy remains within (sub) microliters. The true aspirated and dispensed volumes are unknown, so improperly calibrated pipettes can significantly reduce the reproducibility of experiments, leading to inaccurate published protocols.
The experienced service team and strong production support team provide customers with worry-free order services.

简体中文

繁體中文

English

日本語

한국인