-
article · 2026Year59Moon20Day
为什么选择 Opentrons Flex 高通量 NGS 工作站?
Read More -
article · 2026Year52Moon20Day
Opentrons Flex 高通量 NGS 工作站详解:功能与优势介绍
Read More -
article · 2026Year17Moon19Day
Flex蛋白质组学工作站的自动化技术如何提升实验室效率?
Read More


Author
BO person Lin,PhD1,kin day Watson,PhD 1open TR ONS lab works,Inc.
AbstractThis study conducted an automated magnetic bead immunoprecipitation test on the Opentrons OT-2 automated pipetting workstation. The experimental process can handle up to 96 samples and minimize manual operation time. . We dilute the recombinant GAPDH protein with lysis buffer or directly use HEp-2 cell lysate for sample preparation, and couple it to protein G or protein A, and then perform immunoprecipitation experiments on Thermo Fisher Dynabeads (protein G and protein A can be combined with soluble The antibody binds, thereby promoting subsequent binding to the target protein GAPDH). The protein analysis results showed that the target protein was successfully extracted from the sample solution and was consistent with the expected results, proving that OT-2 has the ability to automate immunoprecipitation experiments.
IntroductionImmunoprecipitation is a common experimental method that uses antibodies to capture target proteins in a mixture of molecules. Antibodies can be pre-linked to a solid-phase matrix (such as agarose resin or magnetic beads) and then contacted with the target protein, or added directly to the corresponding solid-phase matrix together with the sample. Since the incubation time depends on the affinity of the antibody to the target protein, different target capture proteins will require different incubation times. Using magnetic bead extraction, the antibody-protein-magnetic bead complex is precipitated and collected by centrifugation or magnetism.
DynabeadsM (Thermo Fisher Scientific, USA) is a superparamagnetic bead that can be adjusted according to needs to obtain different functions. For example, recombinant protein G or protein A can be covalently bound to the magnetic bead surface. Protein G and protein A are bacterial proteins that exhibit high affinity for the Fc region of monoclonal or polyclonal IgG-type antibodies. DynabeadsTM-Protein G or DynabeadsTM-Protein A can be used as solid support materials for antibodies. Opentrons has designed an efficient and automated experimental program based on DynabeadsM for immunoprecipitation experiments. Each experiment can handle up to 96 samples and has good repeatability between samples.
Materials and MethodsThe experimental steps include the following: the first part is the mixing preparation of magnetic beads, antibodies and samples; the incubation time of the antibody/target protein determined by the user; the second part is the washing and elution steps (Figure 1). The first and second parts were performed on the OT-2, using the magnetic bead purification module to separate the magnetic beads from the solution, and using the temperature control module to prepare the eluate of the denatured protein for SDS-PAGE. The incubation process is performed on a thermal shaker (optional). The OT-2 loading layout for the first and second parts of the immunoprecipitation experiment is shown in Figure 2. This procedure is designed to transfer reagents directly from 15 mL centrifuge tubes to the work plate for magnetic bead separation. Additionally, experiment time can be reduced by using automated procedures to prefill reagent plates on the OT-2. The binding of the antibody to the target protein is the most critical step for successful immunoprecipitation, which depends on the affinity of the antibody. For antibodies with low binding affinity or low abundance, preincubation in sample solution is recommended.

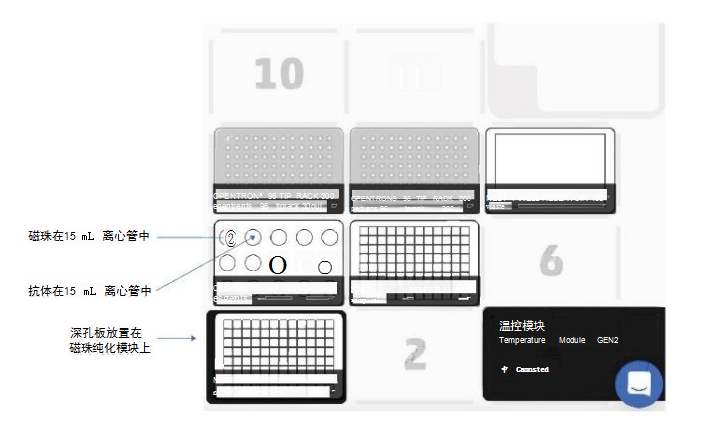
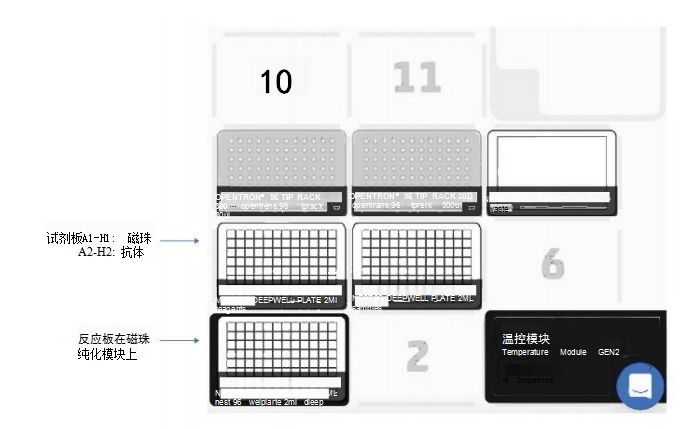
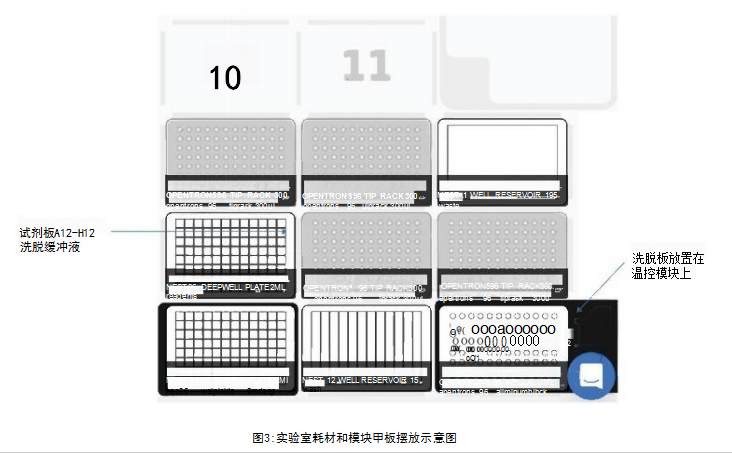
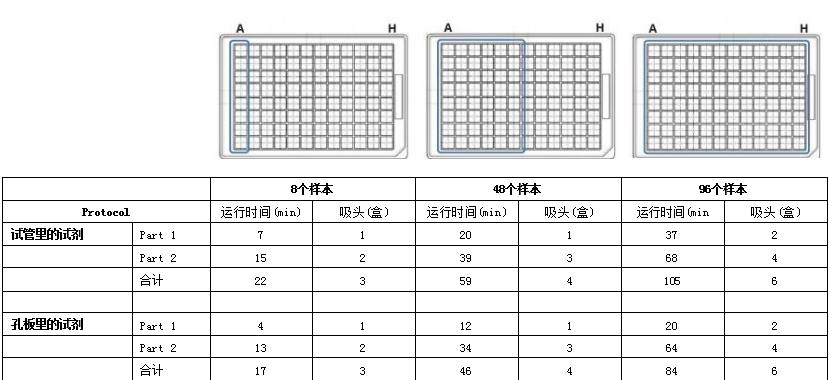
ResultsRabbit IgG has high affinity for both protein G and protein A. To confirm the interaction of the antibody with DynabeadsM, we used anti-GAPDH polyclonal gG antibody (Proteintech Rosemont, IL, USA) . On OT-2, mix 50 μL of magnetic bead suspension with 200 μL of PBS containing 2.5 μg of GAPDH antibody, then mix on a shaker at 800 rpm for 10 minutes at room temperature. Subsequently, the OT-2 was washed three times with 0.1% Tween 20 in phosphate-buffered saline (PBS-T). Bound GAPDH antibodies were eluted by heating at 70°C for 10 minutes and using Laemmli sample buffer (Bio-Rad, USA). Then, they were separated by SDS-PAGE and probed with a goat anti-rabbit IgG antibody (LI-COR Biosciences, USA) coupled with a near-infrared luminescent dye (IRDye 680RD). Western Blot analysis results showed that when repeated four times, both protein G and protein A magnetic beads were able to bind to the GAPDH antibody (Figure 5A), and the binding was reproducible. For immunoprecipitation of GAPDH, we performed immunoprecipitation on OT-2 using samples containing recombinant human GAPDH protein (rhGAPDH, Thermo Fisher Scientific, USA). Tested simultaneously using Dynabeads™-Protein G and Protein A-conjugated GAPDH antibodies. Western Blot results showed the presence of GAPDH, verifying that OT-2 has the ability to run automated immunoprecipitation (Figure 5B). This method was also evaluated for automated immunoprecipitation of endogenous GAPDH in cultured cells. HEp-2 cells were cultured in GibcoTM minimal basal medium (Thermo Fisher Scientific, USA) containing 10% fetal calf serum and 1% penicillin-streptomycin at 5% CO2 and 37°C. To prepare cell lysates for testing, 4 x106 HEp-2 cells were collected and lysed in 200 μL of PierceTM IP lysis buffer plus a protease inhibitor cocktail from Thermo Fisher Scientific. DynabeadsTM-Protein A was used to perform immunoprecipitation experiments on the OT-2 platform. The OT-2 pipetting platform is equipped with a magnetic bead purification module and a temperature control module. In order to allow sufficient time for the antibody to bind to the target protein, place the mixture on a thermal shaker at 4°C and incubate at 800 rpm overnight. Finally, Western Blot quantification was performed, and the results further proved that this immunoprecipitation experiment was successful and had good repeatability (Figure 6). This experiment processed 96 samples. The immunoprecipitated cell lysate was loaded in the last column of the 96-well sample plate, while the remaining positions of the sample plate were only loaded with lysis buffer.
ConclusionOT-2 has good capabilities and practicality for automated immunoprecipitation using Dynabeads in medium and high-throughput workflows. The application protocol we developed replaces manual procedures and reduces hands-on time while providing excellent reproducibility.
Original address:Immunoprecipitation using Thermo Fisher Dynabeads on OT-2
The experienced service team and strong production support team provide customers with worry-free order services.

简体中文

繁體中文

English

日本語

한국인