Flex应用专题 | 解锁蛋白质谱前处理自动化的无限潜能
Check the Details-
article · 2025Year3Moon9Day
清洗微孔板的具体步骤是什么
Read More -
article · 2025Year56Moon8Day
磁珠分选是什么
Read More -
Press release · 2025Year40Moon8Day
云端相约 | 邀您共同解锁蛋白质谱前处理自动化无限潜能
Read More
In the broad fields of biochemistry and molecular biology, protein purification is a crucial technology, which aims to accurately extract and purify a single target protein from complex biological samples. This process not only requires a high degree of precision, but also takes into account the physicochemical properties of the protein and the protection of its biological activity. In order to purify proteins more quickly and conveniently, scientists have developed various protein purification methods:
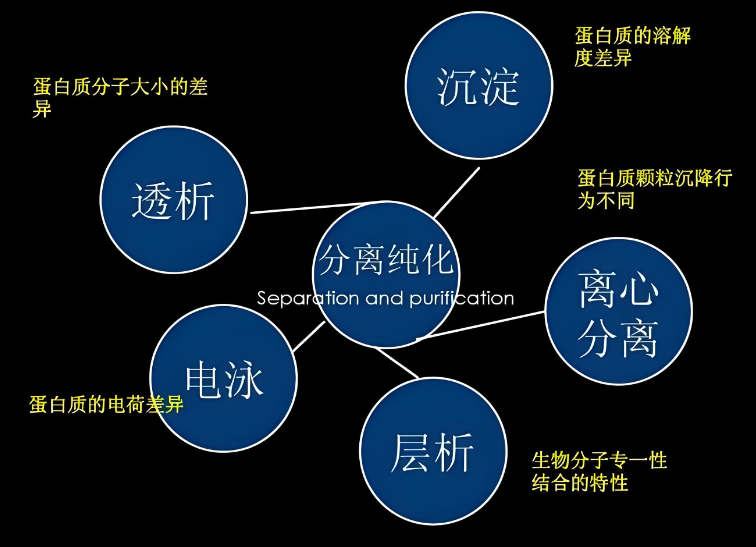
1. Salting out method
Principle: The salting out method is to add high concentrations of neutral salts (such as ammonium sulfate, sodium sulfate, etc.) to the protein solution to reduce the solubility of the protein in water and precipitate out. The ions produced by salt dissociation compete for most of the free water in the solution, thereby destroying the hydration of the protein, reducing its solubility, and causing protein precipitation. In addition, the dissociation of salt can also inhibit the dissociation of weak protein electrolytes, making the protein less charged and more likely to aggregate and precipitate.
2. Dialysis and ultrafiltration
principle:
1. Dialysis: Use a semipermeable membrane that can only pass through small molecule compounds to separate macromolecular proteins from small molecule compounds (such as salts, small molecule metabolites, etc.) to achieve the purpose of concentrating proteins or removing small molecule impurities.
2. Ultrafiltration: Under a certain pressure, small molecular solutes and solvents are allowed to pass through a special membrane (ultrafiltration membrane) with a certain pore size, while large molecular solutes (such as proteins) are retained, thereby achieving preliminary concentration and purification of proteins. .
3. Electrophoresis method
Principle: Proteins in an electric field will migrate due to differences in charge and molecular weight, forming different electrophoretic bands. By adjusting electrophoresis conditions (such as electric field strength, buffer pH, etc.), protein separation and purification can be achieved. Commonly used electrophoresis methods include SDS-polyacrylamide gel electrophoresis (SDS-PAGE), isoelectric focusing electrophoresis and two-dimensional gel electrophoresis.
4. Chromatography (chromatography)
Principle: Chromatography is a method that uses the different distribution coefficients of each protein molecule between the stationary phase and the mobile phase to achieve separation. Depending on the separation principle, chromatography can be divided into many types, including:
1. Gel filtration (molecular sieve chromatography): Separates proteins based on differences in molecular size. The chromatography column is filled with particles with small holes (such as dextran gel). Small molecule proteins can enter the interior of the particles, but large molecule proteins cannot. Therefore, proteins with different molecular weights have different retention times in the chromatography column, thus Achieve separation.
2. Ion exchange chromatography: Separate proteins based on their charge differences. The chromatography column is filled with charged resin, and charge exchange occurs between the protein and the resin. By changing the ion concentration or pH value of the buffer, the protein is separated.
3. Affinity chromatography: Use the high affinity between the target protein and a specific affinity agent (such as antibodies, ligands, metal ions, etc.) for separation. The affinity agent is fixed on the chromatography column, and the target protein is retained on the column after binding to the affinity agent. By changing the elution conditions (such as pH value, adding specific ligands, etc.), the target protein is desorbed and collected.
5. Ultracentrifugation
Principle: Separate proteins based on density and morphological differences. Under the action of ultracentrifugal force, proteins of different densities and shapes will form different sedimentation bands in the centrifuge tube, thereby achieving separation. Ultracentrifugation is commonly used to separate organelles of different sizes and densities as well as proteins in subcellular fractions.
6. Other methods
In addition to the above methods, there are also various protein purification methods such as isoelectric point precipitation, organic solvent precipitation, countercurrent chromatography, and hydrophobic interaction chromatography. Each of these methods has advantages and disadvantages and is suitable for different types of protein purification needs.
Protein purification is a complex and delicate process, which requires selecting an appropriate purification method or combining multiple methods for step-by-step purification based on the properties of the target protein and experimental requirements. By continuously optimizing purification conditions and technical means, high-purity and highly active target proteins can be obtained, providing strong support for subsequent biological research and applications.
The experienced service team and strong production support team provide customers with worry-free order services.