Flex应用专题 | 解锁蛋白质谱前处理自动化的无限潜能
Check the Details-
article · 2025Year3Moon9Day
清洗微孔板的具体步骤是什么
Read More -
article · 2025Year56Moon8Day
磁珠分选是什么
Read More -
Press release · 2025Year40Moon8Day
云端相约 | 邀您共同解锁蛋白质谱前处理自动化无限潜能
Read More
In the medical field, the terms "medical device" and "medical device" are often used, but there are some key differences between them. Understanding these differences is important for medical professionals, patients, and regulators in related industries. This article will explore the differences in the definitions, classifications, uses, and regulatory requirements of medical devices and medical devices.
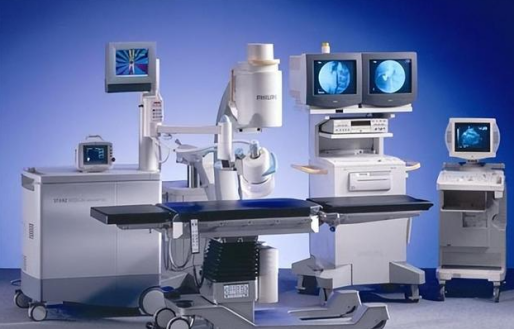
1. Introduction to definition differences
2. Classification and purpose distinction
3. Differences in regulatory requirements
4. Market access and certification
5. Technological Innovation and Development Trends
6. Risk Management and Patient Safety
Although medical devices and medical equipment both play important roles in the medical field, they have obvious differences in terms of definition, classification, regulatory requirements, market access and technological innovation. Understanding these differences can help medical professionals make more appropriate device choices, regulatory agencies develop more effective regulatory strategies, and patients better understand the medical products they use. As medical technology continues to advance, the boundaries between medical devices and medical equipment may become blurred, but their contribution to improving the quality of medical services and patient care cannot be ignored.
The experienced service team and strong production support team provide customers with worry-free order services.